Background: In phase 1 (NCT02407080) and phase 2 (NCT03287245) clinical trials, idasanutlin, a human double minute 2 homolog (HDM2) antagonist, demonstrated robust clinical activity and rapid reduction in JAK2V617F variant allele frequencies (VAF) in patients with hydroxyurea-intolerant or refractory polycythemia vera (PV). Despite promising disease-targeted clinical efficacy, high rates of discontinuation due to toxicity, and concerns regarding the potential clinical implications of transient TP53 mutations had dampened the enthusiasm for pursuing further development of this class of drugs in PV patients. 1,2,3
Given the significant reduction in JAK2V617F VAF that was documented in the clinical trials, we postulated that idasanutlin may have durable clinical effects in terms of reduction in phlebotomy requirement, splenomegaly, and disease progression, as well as questioned the significance of the transient increments in TP53 mutations. To assess the long-term implications of this therapy on PV patients, we examined the longer-term effects of idasanutlin administration.
Methods: We retrospectively evaluated the long-term outcomes of patients enrolled in the phase 1 and 2 clinical trials and used descriptive statistical analysis to evaluate a change in phlebotomy requirements, change in spleen size, and JAK2V617F VAF.
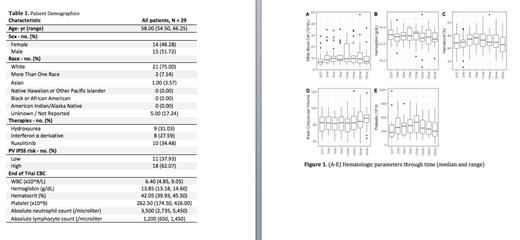
Results: Seventy-six percent (29/38) of the patients enrolled in the phase 1 and phase 2 studies continued to be followed at the participating institutions after the end of trial (EOT) visit (Table 1). Median follow-up after EOT to last follow up visit was 40 months (range XX-XX months). There was no increase in 8-week phlebotomy requirements when comparing 8-week phlebotomy requirement prior to EOT to pre-specified time-points of 1, 3, 6, 12, 18, 24, 36 months after idasanutlin discontinuation (P=.50). Six of 29 (21%) participants remained off PV-directed therapy for the duration of follow up. Therapies initiated in the remainder of the patients are listed in Table 1, four of whom received either combination or sequential therapies. Reduction in splenomegaly with idasanutlin was durable with no observed increase in spleen size by palpation from EOT through the 36-month follow up visit (P=.51). Change in hematologic profile of patients throughout follow up is shown in Figure 1 (median, range).
There were four (13.7%) documented cases of disease progression. Three progressed to myelofibrosis (MF), one to blast phase (BP). Two participants that progressed to MF went on to receive allogeneic hematopoietic cell transplant. Of those who progressed, median time to progression was 17.5 months (range 14-24 months) after treatment discontinuation. Of those that progressed, mutations by next generation sequencing were noted in JAK2 (4), TET2 (2), DNMT3A (1), SF3B1 (1), and ASXL1 (1). None had a known TP53 mutation at the time of disease progression. There were no documented cases of thrombotic events, or hemorrhagic events within the follow-up period. Changes in JAK2V617F VAF after treatment discontinuation will be presented at the meeting.
Conclusion: HDM2 antagonist therapy represents a rational and highly effective treatment approach in PV. The durable effects of idasanutlin on phlebotomy requirements and splenomegaly persist even after treatment discontinuation. Additionally, the lack of appearance of TP53 mutations or loss of TP53 in those who progress suggest that the previously-described transient TP53 mutations do not have long-term clinical implications. Alternative dose and scheduling approaches that are more tolerable may be warranted to safely maintain long-term PV disease control.
1 Mascarenhas J, Passamonti F, Burbury K, et al. The MDM2 antagonist idasanutlin in patients with polycythemia vera: results from a single-arm phase 2 study. Blood Advances. 2022;6(4):1162-1174. doi:10.1182/bloodadvances.2021006043
2 Mascarenhas J, Lu M, Kosiorek H, et al. Oral idasanutlin in patients with polycythemia vera. Blood. 08 08 2019;134(6):525-533. doi:10.1182/blood.2018893545
3 Marcellino BK, Farnoud N, Cassinat B, et al. Transient expansion of TP53 mutated clones in polycythemia vera patients treated with idasanutlin. Blood Advances. 2020;4(22):5735-5744. doi:10.1182/bloodadvances.2020002379
Disclosures
Hoffman:Dexcel Pharma: Research Funding; Curis: Research Funding; Summitomo: Research Funding; Dompe: Patents & Royalties; TD2: Research Funding; Karyopharm: Research Funding; Kartos Abbvie: Research Funding; Silence Therapeutics: Consultancy; Cellinkos: Consultancy; Protagonist Therapeutics: Consultancy. Yacoub:Incyte Corporation: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; CTI Pharma: Consultancy; Pharmaessentia: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Gilead: Consultancy; Apellis: Consultancy; Acceleron Pharma, Inc.: Consultancy; Servier: Consultancy; Notable Labs: Consultancy; AbbVie Inc.: Consultancy; AbbVie, Acceleron, Apellis, CTI Pharma, Gilead, Incyte, Notable Labs, Novartis, Pfizer, PharmaEssentia, Servier.: Consultancy. Gerds:AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy; Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding. Gupta:Novartis, BMS Celgene, SMP Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy; GSK: Other: Travel to EHA 2023 for invited talk at GSK sponsored MPN education session ; BMS Celgene, Roche, AbbVie, Pfizer, Sierra Oncology, CTI Biopharma, GSK: Other: Participation on a Data Safety Monitoring Board or Advisory Board; BMS, Celgene, Roche, Abb Vie, Pfizer, Sierra Oncology, CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis, BMS Celgene, GSK: Honoraria; Novartis, BMS Celgene, Sierra Oncology, AbbVie, Constellation Biopharma, Pfizer, GSK Pharma, CTI Biopharma: Consultancy. Mascarenhas:Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; GSK: Honoraria; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal